The Hardest Job in the World is Running a Biotech
Here's why, and what we can do about it
My core belief, and the first line on my personal website, is simple:
The hardest-working people should work on the hardest problems.
For me, that problem set is in health. Right now, some of the hardest problems in health are in healthcare and drug development.
Few human endeavors combine as much uncertainty, intellect, and consequence as bringing a new therapy to life. It is science, business, operations, and regulation, all colliding in real time, under extreme pressure, with billions of dollars and human lives in the balance.
I learned this firsthand running Healis Therapeutics, where I became the youngest cofounder and leader of a clinical-stage neuroscience therapeutics company in the world. I had to make decisions that even seasoned executives lose sleep over. Every day, I faced questions that had no single right answer: Which indication should we prioritize? How will regulators interpret our trial design? What data matters most to investors, or to patients?
Each decision was a bet made on imperfect information. Like Leonard Schleifer, Founder & CEO of Regeneron, once said: “In biotech, you have to be an optimist who’s also terrified. You’re betting on biology, and biology doesn’t read your business plan.”
To develop a drug is to live in uncertainty, where the data is incomplete, the incentives misaligned, and the timeline unforgiving.
The Four Dimensions of Difficulty
Drug development sits at the intersection of four domains, each complex on its own, but nearly impossible when combined.
1) Biology: she who humbles everyone who touches it
It is the most complex system in the known universe, nonlinear, adaptive, and full of emergent behavior. No two PhDs in biology share the same foundation; even two cancer biologists might struggle to talk meaningfully about each other’s work. One studies DNA repair, another immune signaling, and their worlds barely overlap.
That’s the paradox of modern biology: the deeper you go, the narrower your tunnel becomes. Knowledge diverges faster than it converges. Every discovery spawns ten subfields, and every model works only under certain assumptions. So when a CEO makes a “scientific” decision, they are triangulating across dozens of experts, each seeing a fragment of a reality too large for any single human mind.
2) The market, where uncertainty meets capital
A biotech CEO must understand not just biology, but risk pricing. Every molecule competes not only with disease but with time, capital, and investor psychology. You must decide which indications are viable, which patents are defensible, and which milestones can sustain valuation.
Biotech markets are inherently inefficient because the underlying information is probabilistic and opaque. Even the most sophisticated investors can’t truly know which drug will succeed. That’s why volatility is extreme, and fortunes are made or lost overnight.
Vas Narasimhan, CEO of Novartis, best summed it up: “You can have the best science in the world, but without a sustainable business model, it’s just an academic exercise.”
3) Clinical Trial Operations: a miracle of coordination
Dozens of sites, hundreds of patients, thousands of variables, and everything must align perfectly. Enrollment stalls, data pipelines glitch, CROs misinterpret protocols, or a single manufacturing deviation triggers a cascade of delays. The smallest operational error can destroy months of progress and millions in value.
“In biotech, the science will humble you, but operations will kill you” in the words of John Maraganore, Founding CEO of Alnylam Pharmaceuticals
4) Regulatory: the labyrinth
The FDA and EMA are not adversaries, they’re stewards of trust, but they speak a language of precedent and proof. Success depends not just on what your data shows, but on how it is framed, contextualized, and justified. The path to approval is paved with nuance: every phrase in a briefing document, every comparator chosen, every safety flag raised.
Is biotech really exceptionally difficult?
But doesn’t every CEO face operational, market, and regulatory challenges? Biotech is no exception, right? But in this field, the complexity runs far deeper. The science itself poses some of the most difficult decisions: navigating the intricate nature of biology, managing the operational challenges of conducting precise clinical trials, and sustaining the immense financial investment required for research and development. On top of that, biotech operates within one of the most tightly regulated environments, from the FDA to market approval, involving countless stakeholders. These include governments, insurers, healthcare providers, and more. All of whom must align before the ultimate customer, the patient, can benefit. Unlike most industries, you cannot simply launch a product based on consumer demand or willingness to pay. The path to market in biotech is a winding one, with few clear roadmaps and limited opportunities to learn from past examples.
Living in a World of Uncertainty
When you combine these four layers, scientific, business, operational, and regulatory, you get a system so complex it borders on chaotic. Each layer has its own data, incentives, and epistemology. No one, not the scientists, not the investors, not the regulators, can see the entire picture.
That’s why biotech is still, in many ways, an era of intelligent gambling. We make our best bets with partial data and hope that biology cooperates. The result is staggering inefficiency. Over 90% of drugs that enter clinical trials fail. Biotech stock prices swing by 50–80% on single readouts. And even “experts” often disagree on which programs will succeed or why.
This isn’t because people are careless, it’s because the information space is fractured. The human mind can’t integrate the full spectrum of molecular, clinical, operational, and economic signals simultaneously. Every decision is filtered through the narrow bandwidth of human cognition and the silos of expertise.
The outcome is a landscape defined by uncertainty and inefficiency, one where insight is scattered, coordination is imperfect, and progress comes at enormous cost.
“If you can’t live with ambiguity, you can’t live in biotech.”
- Bob Langer, Co-founder of Moderna
When It Works, and When It Doesn’t
When it goes well, it’s a masterpiece of coordination and courage.
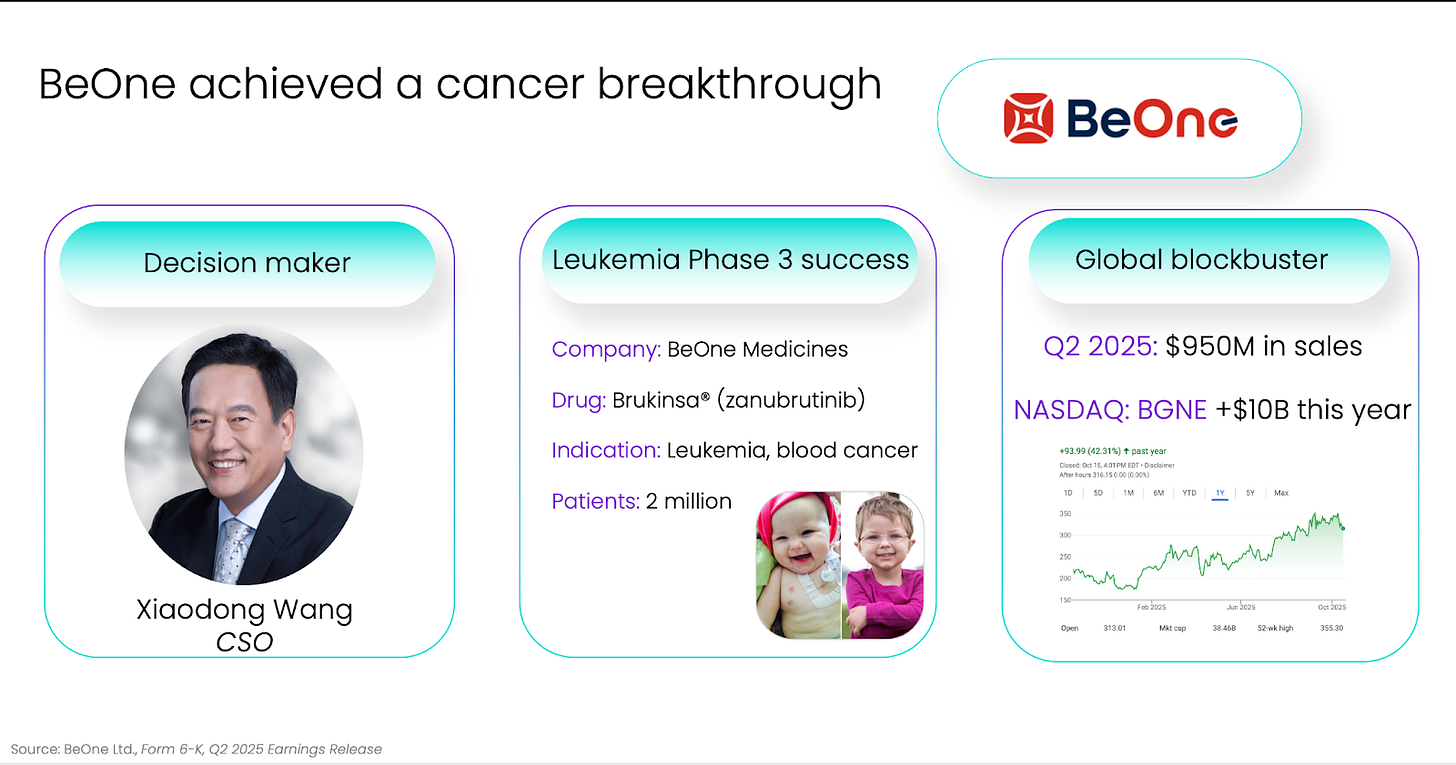
Look at BeOne’s success in leukemia, a precise scientific bet, executed with operational discipline, aligned with regulators and investors alike.
Billions in value created, and many lives saved. A cancer breakthrough to marvel at.
But when it goes wrong, it’s devastating.
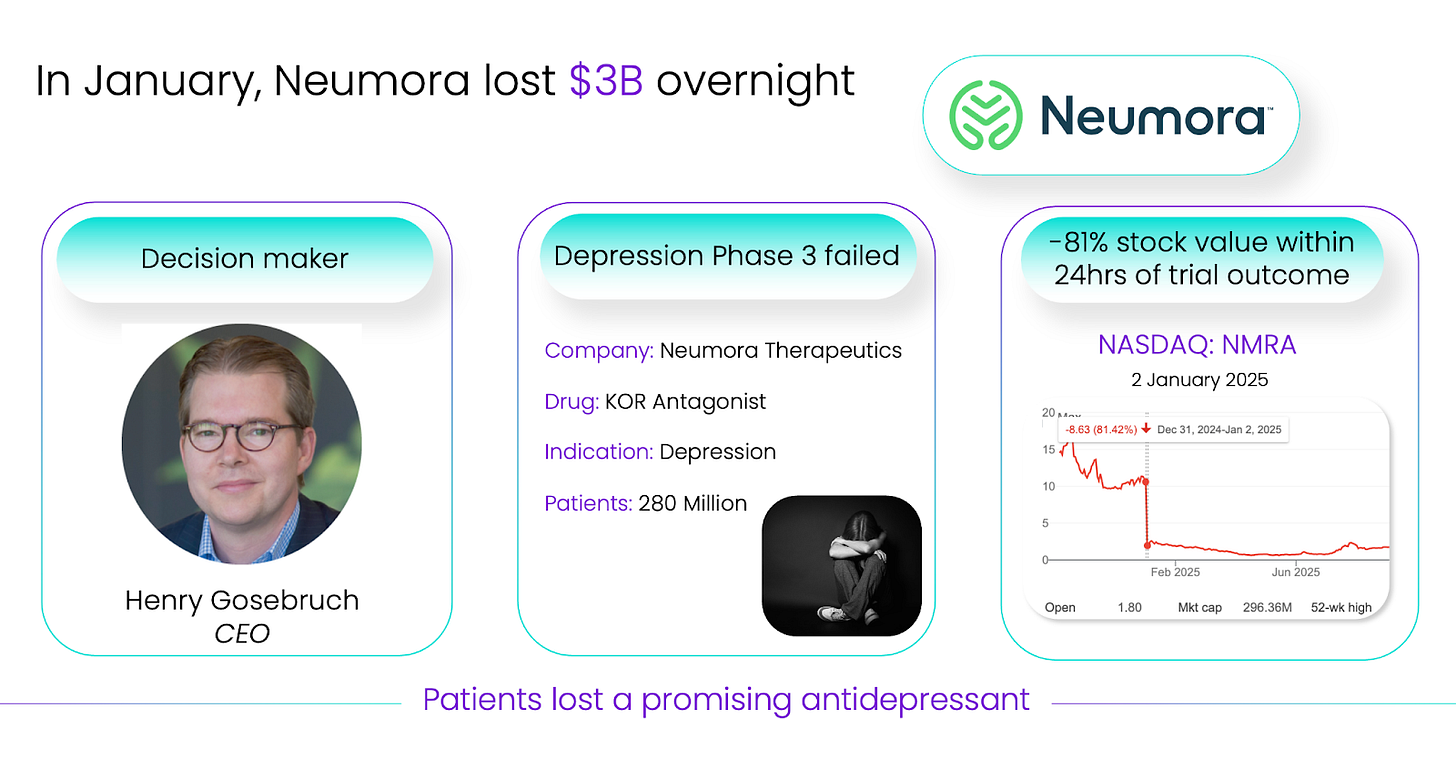
Think of Neumora’s failed Phase 3 in depression, a single trial readout that erased $3 billion in market cap overnight. The same molecule, the same scientists, the same commitment, but biology said no.
This is exactly why I believe running a biotech is the hardest job in the world.
You must be fluent in four languages, scientific, commercial, operational, and regulatory, and translate between them every day. You must hold conviction under uncertainty and humility under success.
The Opportunity Hidden in the Chaos
But amid this chaos lies opportunity, because chaos has structure, if you can find it.
The inefficiency of biotech markets isn’t just a risk; it’s a signal. The noise hides patterns, correlations between trial design, mechanism, regulatory precedent, and real-world outcomes. The data is there; it’s just fragmented, unstructured, and uncalibrated.
What if we could make sense of it?
What if we could collect, integrate, and annotate every piece of trial data, mechanistic knowledge, regulatory precedent, and market outcome, and train on it?
What if we could develop an intelligence that doesn’t just memorize, but reasons, across domains, across silos, an intelligence that can see connections no single human can?
A Clinical Trial Intelligence:
Trained on the world’s biomedical, operational, and market data,
Calibrated to the real-world success and failure of clinical trials,
Capable of making cross-domain decisions like the collective mind of 10 million experts.
An agent that thinks like a scientist, operates like an executive, and reasons like a regulator.
A synthetic super life sciences executive, one that can simulate, predict, and optimize across the hardest problems in the world.
Because what this industry needs isn’t more data.
It needs understanding. And understanding comes from learning.
The Future of Biotech Leadership
The future of drug development will belong to those who can integrate, not just specialize. The leaders of tomorrow will partner with systems that think alongside them, not replacing human expertise, but amplifying it.
If we can build an intelligence that bridges biology, business, operations, and regulation, that makes sense of uncertainty and discovers the hidden structure in the chaos, then perhaps, for the first time, drug development won’t feel like gambling.
It will feel like progress.
And maybe then, the hardest-working people in the world will finally have the tools they deserve, to move the hardest problems in the world, faster and further than ever before.
Sources:
IQVIA Institute. The Global Use of Medicines: Outlook to 2028 (Jan 2024) https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/the-global-use-of-medicines-2024-outlook-to-2028 IQVIA+2IQVIA+2
Biotechnology Innovation Organization (BIO). Clinical Development Success Rates 2011–2020 https://www.bio.org/clinical-development-success-rates-and-contributing-factors-2011-2020 BIO+1
ScienceDirect / Elsevier. Benchmarking R&D Success Rates of Leading Pharmaceutical Companies https://www.sciencedirect.com/science/article/pii/S1359644625000042 ScienceDirect+1
Alzheimer’s Disease International (ADI). World Alzheimer Report 2022 https://www.alzint.org/resource/world-alzheimer-report-2022/ Alzheimer’s Disease International+1
JAMA Oncology. Estimates and Projections of the Global Economic Cost of 29 Cancers: 2020–2050 https://jamanetwork.com/journals/jamaoncology/fullarticle/2801798 JAMA Network+1
American Heart Association (AHA). Heart Disease and Stroke Statistics 2024 https://www.heart.org/-/media/PHD-Files-2/Science-News/2/2024-Heart-and-Stroke-Stat-Update/2024-Statistics-At-A-Glance-final_2024.pdf?hash=D0A208F50F8591AEED0E31BE77265505&sc_lang=en www.heart.org+1
United Nations. World Population Prospects 2024 https://population.un.org/wpp/ population.un.org+1