Panoptic Bio’s Clinical Trial Outcome Prediction
Ending the year with a small preview of what we’ve been building
After a period of quiet development, we are sharing a first, high-level result from our clinical trial outcome prediction work. Our goal is simple: help teams make clinical trial decisions with greater confidence and lower risk.
Our clinical trial prediction model predicts the probability of phase transition, not just a relative ranking of trials. For example, transitioning from Phase I to Phase II, from Phase II to Phase III, or from Phase III to Approval.
We evaluate both discrimination (how well the model separates successes from failures) and calibration (how closely predicted probabilities match observed outcomes).
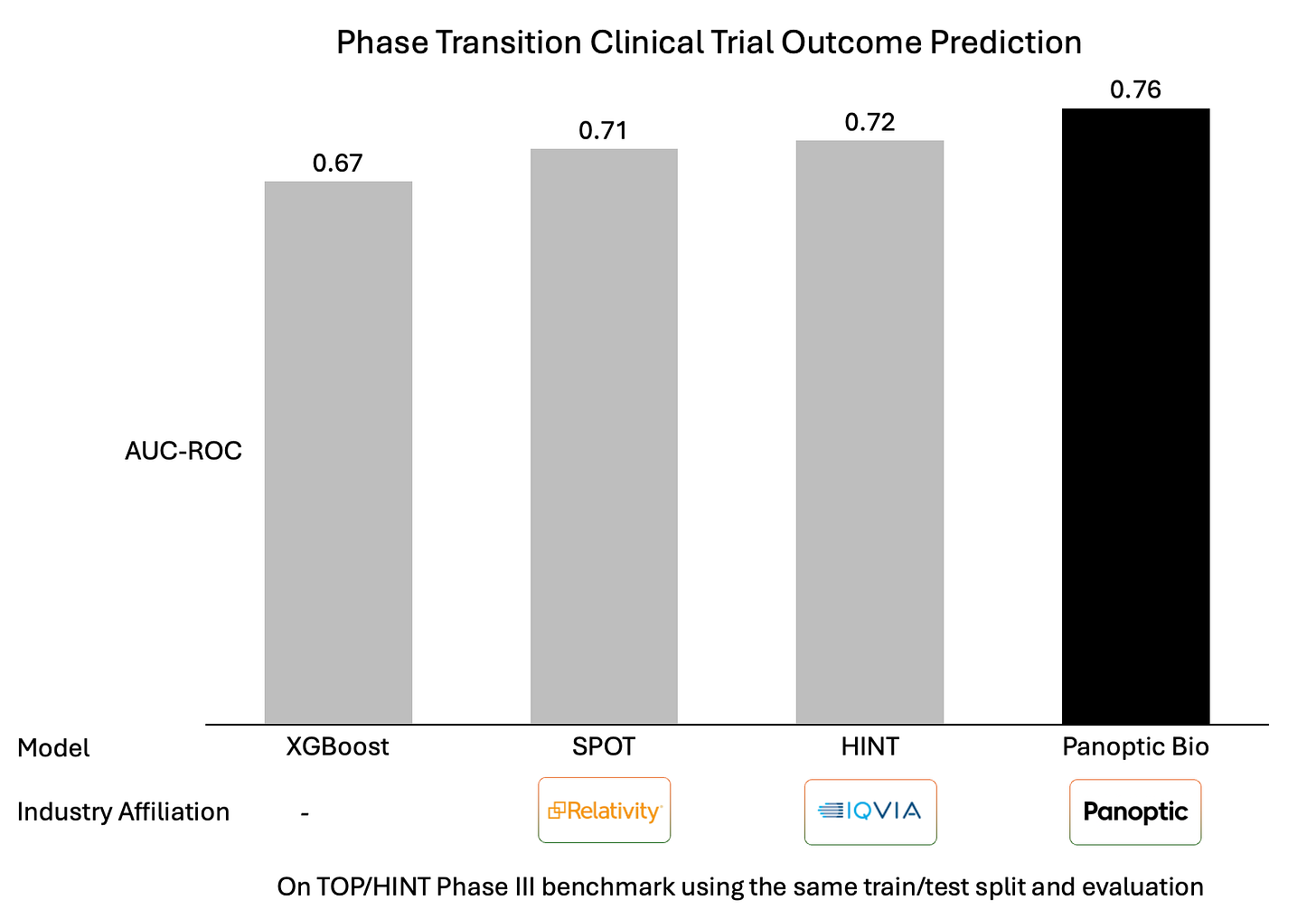
Benchmark Performance
On the widely used TOP/HINT benchmark, evaluated using the same train/test split and evaluation protocol as published baselines, our Panoptic Bio model achieved an ROC–AUC of 0.76.
Our result reflects improved discrimination on late-stage trials under a fixed evaluation metric.
OpenAI and Babylon Biosciences
This year, media reports described a collaboration between OpenAI and Babylon Biosciences in which a clinical trial prediction model trained on 430 historical clinical trials reported an AUC of approximately 0.84. These results have not appeared in a peer-reviewed publication and were reported through media coverage. It is also important to note that the dataset of 430 clinical trials was not published, and that there are significant differences in task definition and evaluation metrics compared to the TOP/HINT benchmark.
Panoptic Bio’s evaluation was conducted on a substantially larger corpus, spanning:
11,600 historical clinical trials
560,398 unique documents, including scientific, regulatory, commercial, and operational sources
Predictions are produced as calibrated probabilities with accompanying uncertainty estimates (including confidence intervals and precision metrics), making outputs suitable for direct use in due diligence, portfolio analysis, and go/no-go decisions in clinical development, an area where more than $276B is spent annually.
Access is available through the Panoptic Bio Trial Terminal.
What We Are Sharing vs Not Sharing
We share here:
The definition of the prediction target
The evaluation setup
Benchmark results
We are not publishing:
The full feature set
Training recipes
Our proprietary system architecture details
We are happy to share further technical evidence with qualified partners and to evaluate externally specified trials so results can be independently verified.
Try It on Your Trial
In the spirit of the holidays, we are offering a limited number of trial evaluations at no cost for select partners in the Panoptic Bio Trial Terminal.
Consider it a free trial. No pun intended.
A Peak Inside the Panoptic Bio Trial Terminal
Our team has worked tirelessly on this. Our lead applied AI scientist was trained by two of the most prominent researchers in the field who created a novel, now famous, lightweight ensemble model. Much gratitude to this milestone and those in our team who supported this early result.
Panoptic Bio, Inc is a pharma intelligence company based in Mountain View, CA